

 So what exactly is vapour permeability?
So what exactly is vapour permeability?Vapour permeability is a material's ability to allow a vapour (such as water vapour or, indeed any gas) to pass through it. To be more precise it is a measure of how much vapour is transmitted through a material (or compound object) under a given set of circumstances. The higher the value of the permeability of the material, the more rapidly vapour can pass through it. Vapour permeability is the rate at which vapour passes through a material.
Technically molecules of a gas or vapour move from regions of higher concentration to regions of lower concentration. This process is driven by the kinetic energy of the molecules.
All materials allow gases to flow, or permeate, through them. The gaseous molecules simply flow, one by one, through the atomic structure of the material and emerge on the other side. Some materials are much better at allowing vapour to permeate or flow through them than others, some make better barriers.
A material does not have a single vapour permeability for all gazes / vapours the transmission rate depend critically on the specific vapour, and sometimes on a vapour's specific isotopes. For example water vapour may flow easily through one material, but that same material may put up much greater resistance to another vapour - such as oxygen, carbon dioxide or a hydrocarbon. The pressure difference of the vapour across the material can also make a big difference, as can temperature. Thus the vapour permeability of a material is given for specific materials at specific temperatures, specific material thicknesses and specific vapour partial pressures.
As Vapour permeability is a measure of the rate of flow, rather than the resistance, when vapour permeability values increase, more and more of that vapour can flow through the material. Similarly lower vapour permeability values reduce the flow of the vapour across the barrier. Flow resistance is the inverse of the vapour permeability.
In many practical cases we are often looking at a materials barrier properties - so that it can keep a gas / vapour either contained inside an enclosure (such as a petrol can or rubber tubing) or keeping it out (such as keeping water vapour out of food or medical products).
There are three parts to the permeation process. First comes absorption where the gas or vapour molecules dissolve into the material's surface. Next is diffusion where the molecules move through the material by diffusion, driven by the concentration gradient. Lastly comes desorption where the molecules emerge from the opposite surface of the material.
Factors Influencing Vapour Permeability
s.
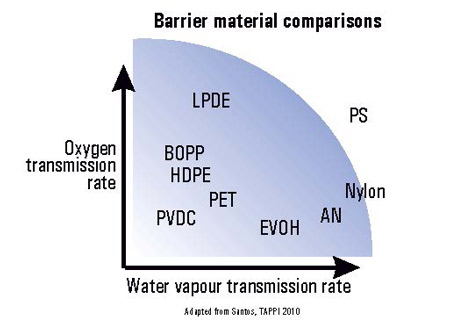
The Maths and science behind Vapour Permeability
To understand the mathematics behind Permeability (Flicks' laws) click here.
For equipment to measure vapour permeability click here
For information on vapour permeability testing click here
For background information on vapour permeability click here
For Processing Talk coverage click here .
For Permeability tester on access my library.com click here
For Water vapour transmission rate on highbeam click here
For Fast accurate measurement of water permeability on Manufacturing Talk click here
For "water water everywhere" on Laboratory Talk click here