

 Fick's First Law states that 'the rate of "diffusion (e.g. of a vapour) is proportional to the surface area and the concentration difference, it is inversely proportional to the thickness of the membrane".
Fick's First Law states that 'the rate of "diffusion (e.g. of a vapour) is proportional to the surface area and the concentration difference, it is inversely proportional to the thickness of the membrane".
This means that the amount of gas transferred per unit time across a membrane is proportional to the area available for exchange and the partial pressure difference of the gas across the membrane. The constant of proportionality (K) is called the-diffusion coefficient. This is the same as saying that the vapour flows from regions of high concentration to regions of low concentration, and the amount that flows is proportional to the concentration gradient across the membrane
The law can be expressed mathematically in many forms, including :-
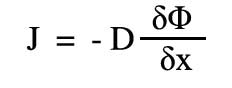
where
Alternatively it can be expressed as
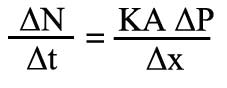
Where:
However Fick can only be applied to systems that are in a steady state and the system is in unchanging. However, measurements can be facilitated by factors that do not effect Fick's law - such as convection air currents, that assist in spreading particles and sweeping them past sensors.
In situations where situations are changing, for example while pressures are being brought into balance, Fick's Second law can be applied.
Fick's 2nd law of diffusion states that the rate of accumulation (or depletion) of concentration within the volume is proportional to the local curvature of the concentration gradient.
It is expressed mathematically as a partial differential equation:-
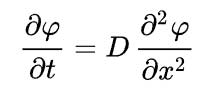
where
In two or more dimensions the Laplacian Δ = ∇2, is required to generalises the second derivative leading to
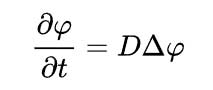
The you tube video ( https://www.youtube.com/watch?v=iOHFrtCxAxc&t=2s )+ is useful in explainning Fick's law made by Texas A&M: Intro to Materials. It is relevant to all fluids such as liquids and gases / vapours.