

 The efficiency, durability and safety of fuel cells are all based around the hydrogen vapour permeability of the fuel cell, and, in particular, the membrane at its heart. Vapour permeability can impact performance by over 500%.and is critical to future developments
The efficiency, durability and safety of fuel cells are all based around the hydrogen vapour permeability of the fuel cell, and, in particular, the membrane at its heart. Vapour permeability can impact performance by over 500%.and is critical to future developments
Hydrogen is the key element, particularly in proton exchange membrane fuel cells (PEMFCs), where it serves as the primary fuel. Because of their high efficiency and environmentally friendly operation, fuel cells have become widely used in applications ranging from portable electronics to vehicles.
Proton Exchange Membrane (PEM) Fuel Cells
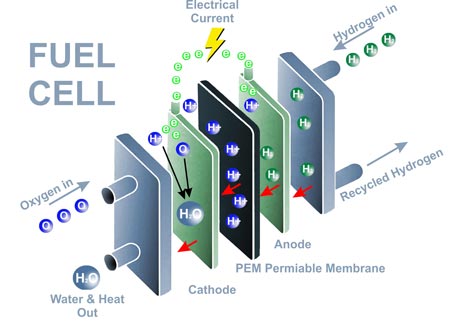
In a PEM fuel cell, hydrogen gas (H₂) is supplied to the anode, where it is split into protons and electrons via a catalytic reaction. The protons pass through a proton-conducting membrane and the electrons need to flow through an external circuit to balance the resulting charge imbalance. Thus generating electricity. The protons then recombine with oxygen at the cathode to form water, which is the only byproduct.
Hydrogen Vapour Permeability in Fuel Cells
Hydrogen vapour permeability is the ability of hydrogen vapour form to pass through, or permeate, through materials, especially the polymer membrane used in PEM fuel cells. This property is critical for several reasons:
 Controlling Hydrogen Permeability
Controlling Hydrogen Permeability
The design and material composition of the membrane are key to controlling hydrogen permeability. The membrane at the heart of the fuel cell is designed to have high proton conductivity while limiting hydrogen crossover. Ongoing research focuses on improving membrane materials to reduce hydrogen permeability still further enhance overall fuel cell performance.
Summary
Hydrogen vapour permeability is critical to the design and operation of PEM fuel cells. It directly affects their efficiency, durability, and safety. Controlling this factor through tested and proven material selection, membrane thickness, and operational parameters is critically impotent to fuel cell performance. As fuel cell technology advances, innovations in membrane materials continue to play the pivotal role in managing hydrogen permeability effectively.